
Structure & Reactivity in Chemistry
Introduction to Molecules
IM5. Lewis and Formal Charges
Looking at the structure of a molecule can help us to understand or to predict the behaviour of that compound. One of the tools that we will eventually use to understand reactivity is formal charge. That is because reactivity has to do with the reorganization of electrons between atoms. New chemical bonds are formed by sharing electrons. Old chemical bonds are broken when one atom takes the bonding electrons away from another atom.
Formal charge can help us to understand the behaviour of carbon monoxide, CO. When exposed to transition metal cations such as the iron in hemoglobin (Fe2+), the carbon is attracted to and binds to the metal. In the case of hemoglobin, because the carbon monoxide binds very strongly to the iron, the CO blocks the position where oxygen would normally be bound and carbon monoxide poisoning results.
Why does the molecule behave in this way? There are actually a number of reasons. However, the fact that the carbon is attracted to a metal cation begs the question: Is the carbon an anion? Yes, in a sense. In a Lewis structure of the compound, the carbon has a formal negative charge. You will see why below.
Formal charges are an important book-keeping device that we use in Lewis structures. They tell us if one atom is donating extra electrons to another to give it an octet. If an atom needs to donate more electrons than normal in order for everyone to get an octet, it will have a positive formal charge. If an atom donates fewer electrons than normal and everyone still has an octet, it must be getting extra electrons from somewhere else. It will have a negative formal charge.
To help us think about formal charges, let's look at a few small molecules that all contain carbon-oxygen multiple bonds but that are slightly different from each other. Formaldehyde (CH2O) is a chemical that is used to preserve tissues; you may be familiar with its odour from anatomy lab. Carbon monoxide results from burning fossil fuels; it is also an important industrial chemical used in manufacturing detergents. Carbonate is an anion that is found in many forms. Calcium carbonate is found in limestone and chalk, for instance.
Look at the structure of formaldehyde. Oxygen has a normal valence of two, and it has two bonds in formaldehyde, so there is no formal charge on the oxygen. Carbon has a normal valence of four, and it has four bonds here. There is no formal charge on carbon. There are no formal charges on the hydrogens either.

Figure IM5.1. The structure of formaldehyde.
Carbon monoxide has a structure that is very similar to formaldehyde. It does not have any hydrogens, though. With ten electrons total, the only way to get an octet on both atoms is to make three bonds between carbon and oxygen.
![]()
Figure IM5.2. The structure of carbon monoxide.
Oxygen has normal valence two, but here it is making three bonds. It is sharing an extra pair of its electrons with carbon to make that third bond. If it is sharing a pair of electrons, we can think of it keeping one for itself and giving the other to carbon. Since it gives one of its electrons to carbon, it has formal charge +1.
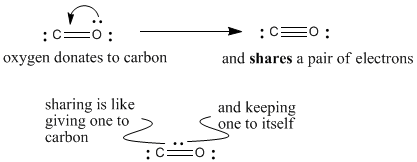
Figure IM5.3. The third bond in carbon monoxide.
Carbon has normal valence four, but here it is only making three bonds, even though it has an octet. How did it get an octet with only three bonds? It got an extra electron from somewhere (the oxygen). It has formal charge -1.
![]()
Figure IM5.4. The complete structure of carbon monoxide includes formal charges.
Notice that overall the carbon monoxide molecule is neutral. Oxygen has a plus charge and carbon has a minus charge. These charges cancel to give an overall neutral molecule.
What we are really doing when we assign formal charge is comparing how many electrons the atom brought with it from the periodic table to how many it has now. If the atom brought four electrons of its own and it is now sharing eight, things are even. It brought four to share and got four from its neighbours in an even trade. If it only brought three of its own and is now sharing eight, it got more electrons than it gave, and it will have a negative charge.
To determine formal charge:
Compare the number of entirely owned valence electrons in the periodic table to those entirely owned by the atom in the molecule.
Remember, electron counting to determine an octet counts all of the bonding and nonbonding electrons equally. It is done simply to determine whether the atom has a noble gas configuration right now. Electron counting to determine formal charge is done to keep track of who has given electrons to whom when making the molecule. If, in getting to an octet, atoms have received more electrons than they have given, their electron/proton ratio has changed, and they become charged.
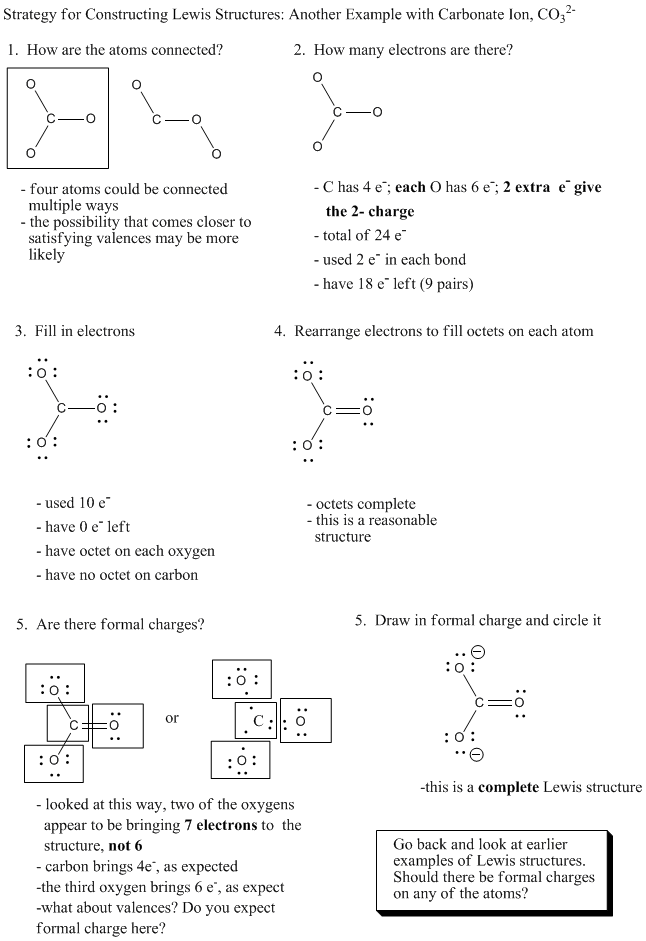
Figure IM5.5. An algorithm for constructing a Lewis structure.
Problem IM5.1
Draw Lewis or Kekule structures for the following molecules, remembering to include formal charges, if any (and notice that some of these molecules are ions):
a) NO+ b) CN- c) CH3O- d) CH3+ e) HNO3 f) CH3CO2-
Problem IM5.2.
Given the structures below, assign any missing formal charges.

Problem IM5.3.
Given the structures below, draw in the missing electrons, if any.

Problem IM5.4.
Label all atoms in the following compounds with the correct non-zero formal charge.
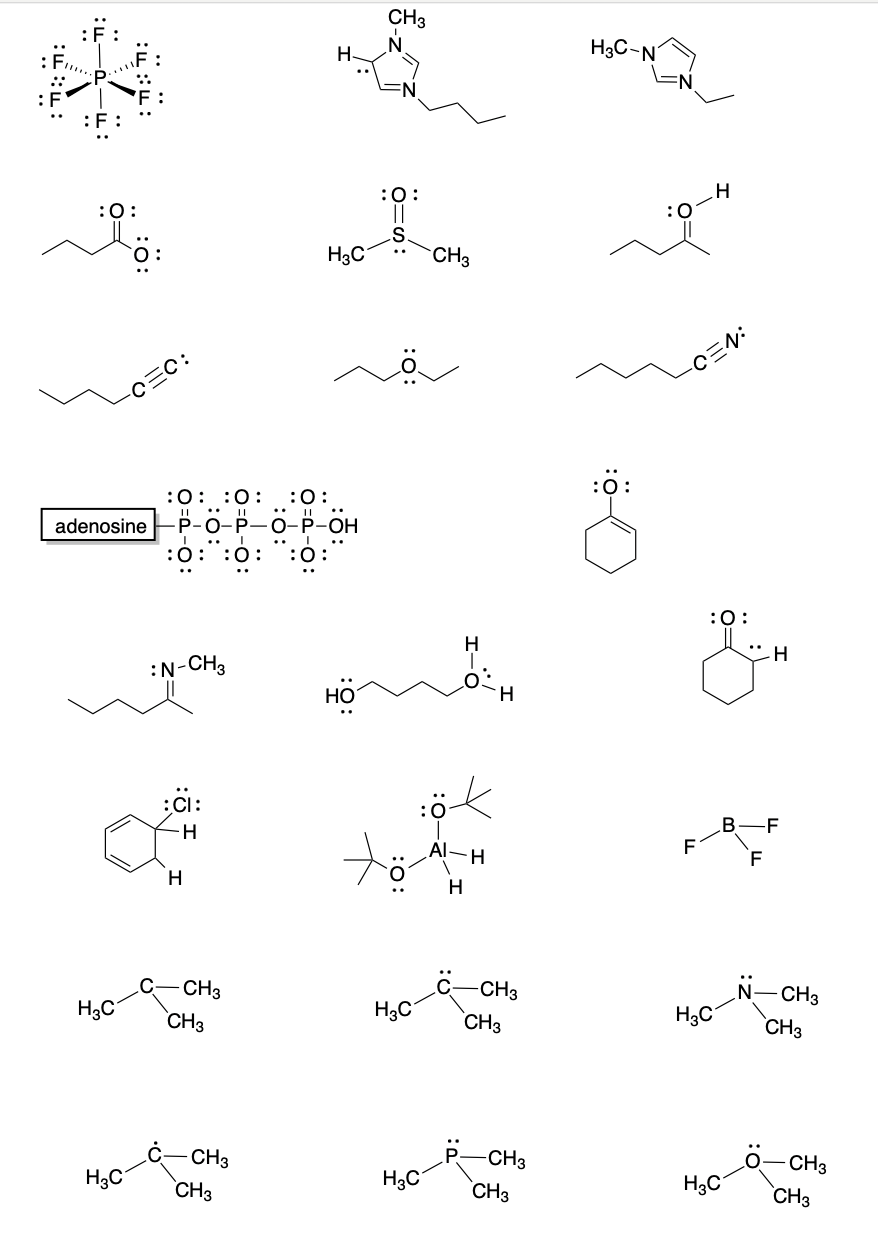
Problem IM5.5.
Provide structures for the following oxoanions (anions with oxygens attached to another atom) of chlorine:
a) hypochlorite, ClO- b) chlorite, ClO2- c) chlorate, ClO3- d) perchlorate, ClO4-
Problem IM5.6.
Provide structures for the following oxoanions of sulfur:
a) sulfite, SO32- b) sulfate, SO42- c) thiosulfate, S2O32- d) disulfate, S2O72- e) persulfate or peroxomonosulfate, SO52-
Problem IM5.7.
Provide structures for the following oxoanions of phosphorus:
a) phosphate, PO43- b) phosphite, HPO32- c) hypophosphite, H2PO2-
Note that hydrogen atoms in some phosphorus oxoanions are attached to the phosphorus atom.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials